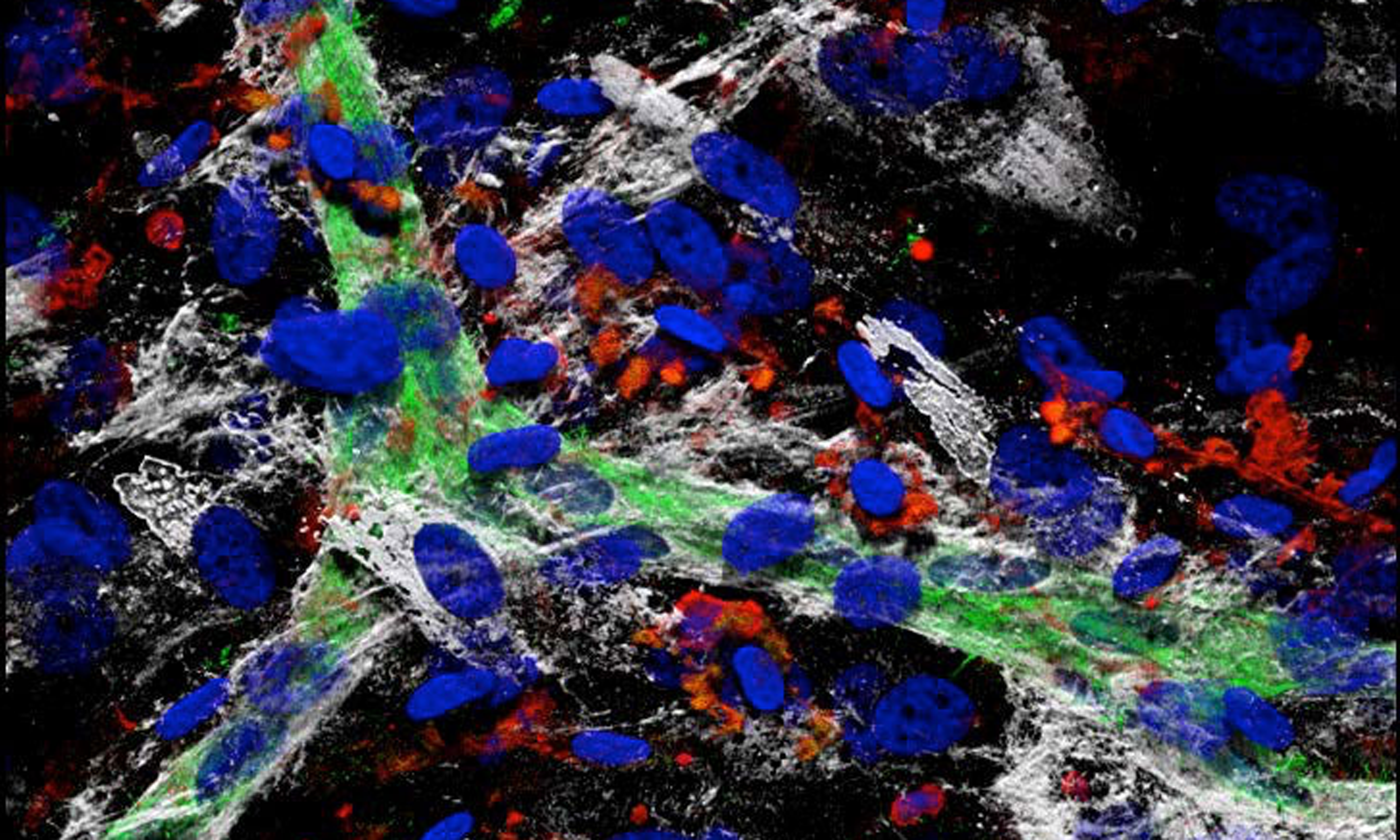
Unser Labor hat zum Ziel Nischenmodelle zu etablieren, um Mechanismen der Zell-Zell- und Zell-Matrix-Interaktionen zu erforschen, welche Regenerationsprozesse nach Gewebeschädigung steuern.

ARin Beate Maria Rüger, MMLSc PhD
wissenschaftliche MitarbeiterinMedizinische Universität Wien
Universitätsklinik für Transfusionsmedizin und Zelltherapie
Währinger Gürtel 18–20, 1090 Wien
Telefon: +43 (0)1 40400-53180
Pager (nur intern verfügbar): 81-1581
E-Mail: beate.rueger@meduniwien.ac.at
- Beate M. Rüger, Tanja Buchacher, Eva-Maria Dauber, Markus Pasztorek, Pavel Uhrin, Michael B. Fischer, Johannes M. Breuss, Gerda C. Leitner (2020). De novo vessel formation through cross-talk of blood-derived cells and mesenchymal stromal cells in the absence of pre-existing vascular structures. Front Bioeng Biotechnol 8:602210. DOI: 10.3389/fbioe.2020.602210
- Nina Kastner, Julia Mester-Tonczar, Johannes Winkler, Denise Traxler, Andreas Spannbauer, Beate M. Rüger, Georg Goliasch, Noemi Pavo, Mariann Gyöngyösi, Katrin Zlabinger (2020). Comparative effect of MSC secretome to MSC co-culture on cardiomyocyte gene expression under hypoxic conditions in vitro. Front Bioeng Biotechnol 8:502213. DOI: 10.3389/fbioe.2020.502213
- Rüger BM, Buchacher T, Giurea A, Kubista B, Fischer MB, Breuss JM (2018). Vascular Morphogenesis in the Context of Inflammation: Self-Organization in a Fibrin-Based 3D Culture System. Front Physiol 9: 679. DOI: 10.3389/fphys.2018.00679
- Haider T, Höftberger R, Rüger B, Mildner M, Blumer R, Mitterbauer A, Buchacher T, Sherif C, Altmann P, Redl H, Gabriel C, Gyöngyösi M, Fischer MB, Lubec G, Ankersmit HJ (2015). The secretome of apoptotic human peripheral blood mononuclear cells attenuates secondary damage following spinal cord injury in rats. Exp Neurol 267: 230-242. DOI: 10.1016/j.expneurol.2015.03.013
- Buchacher T, Wiesinger-Mayr H, Vierlinger K, Rüger BM, Stanek G, Fischer MB, Weber V (2014). Human blood monocytes support persistence, but not replication of the intracellular pathogen C. pneumoniae. BMC Immunol 15(1):60. DOI: 10.1186/s12865-014-0060-1
- Walzer SM, Cetin E, Grübl-Barabas R, Sulzbacher I, Rueger B, Girsch W, Toegel S, Windhager R, Fischer MB (2014). Vascularization of primary and secondary ossification centres in the human growth plate. BMC Dev Biol 14: 36. DOI: 10.1186/s12861-014-0036-7
- David Hollemann, Genya Yanagida, Beate M Rüger, Csilla Neuchrist, Michael B Fischer (2011). New vessel formation in peritumoral area of squamous cell carcinoma of the head and neck. Head & Neck 34(6):813-20. DOI: 10.1002/hed.21814
- Beate M. Rüger, Johannes Breuss, David Hollemann, Genya Yanagida, Michael B. Fischer, Isabella Mosberger, Irene Lang, Andreas Chott, Paul Davis, Paul Höcker, Markus Dettke (2008). Vascular morphogenesis by adult bone-marrow (BM) progenitor cells in three dimensional (3D) fibrin matrices. Differentiation 76:772-783. https://doi.org/10.1111/j.1432-0436.2007.00259.x
- Vaculik C, Rüger BM, Yanagida G, Hollemann D, Soleiman A, Losert UM, Chen J, Fischer MB (2008). Shift of C3 deposition from localization in the glomerulus into the tubulo-interstitial compartment in the absence of secreted IgM in immune complex glomerulonephritis. Clin Exp Immunol 151(1):146-154. DOI: 10.1111/j.1365-2249.2007.03534.x
- Fischer MB, Rüger B, Vaculik C, Becherer A, Wadsak W, Yanagida G, Losert UM, Chen J, Carroll MC, Eibl MM (2007). The presence of MOMA-2+ macrophages in the outer B cell zone and protection of the splenic micro-architecture from LPS-induced destruction depend on secreted IgM. Eur J Immunol 37(10):2825-2833. DOI: 10.1002/eji.200636996
- Giurea A, Rüger B, Hollemann D, Yanagida G, Kotz R, Fischer MB (2006). STRO-1+ mesenchymal precursor cells located in synovial surface projections of patients with osteoarthritis. Osteoarthritis Cartilage 14(9):938-43. https://doi.org/10.1016/j.joca.2006.02.014
- Polgar D, Leisser C, Maier S, Strasser S, Rüger B, Dettke M, Khorchide M, Simonitsch I, Cerni C, Krupitza G (2005). Truncated ALK derived from chromosomal translocation t(2;5)(p23;q35) binds to the SH3 domain of p85-PI3K. Mutation Res 570: 9-15. DOI: 10.1016/j.mrfmmm.2004.09.011
- Rüger BM, Giurea A, Wanivenhaus AH, Zehetgruber H, Hollemann D, Yanagida G, Groger M, Petzelbauer P, Smolen JS, Hoecker P Fischer MB (2004). Endothelial Precursor Cells in the Synovial Tissue of Patients with Rheumatoid Arthritis and Osteoarthritis. Arthritis Rheumatism 50(7): 2157-2166. DOI: 10.1002/art.20506
- Dettke M, Jurko S, Rüger BM, Leitner G, Greinix HT, Kalhs P, Fischer MB, Hocker P (2001). Increased serum flt3-ligand in healthy donors undergoing granulocyte colony-stimulating factor-induced peripheral stem cell mobilization. J Hematother Stem Cell Res 10(2): 317-320. DOI: 10.1089/15258160151135105
- Walker R, Dixon S, Rüger B, Davis P, Hildebrant F, Kremer M, Eccles M, McNoe L, Watson M, Reid J (2001). Familial fibronectin glomerulopathy: analysis of chromosome 1q32 and uteroglobin gene loci in a large New Zealand family. Nephrology 6: 191-197. https://doi.org/10.1046/j.1440-1797.2001.00062.x
- Rüger BM, Erb KJ, He Y, Lane JM, Davis PF, Hasan Q (2000). Interleukin-4 transgenic mice develop glomerulosclerosis independent of immunoglobulin deposition. Eur J Immunol 30(9): 2698-2703. DOI: 10.1002/1521-4141(200009)30:9<2698
- Hasan Q, Rüger BM, Tan ST, Gush J, Davis PF (2000). Clusterin/apoJ expression during the development of hemangioma. Hum Pathol 31(6): 691-697. DOI: 10.1053/hupa.2000.7638
- Tan ST, Velickovic M, Rüger BM, Davis PF (2000). Cellular and extracellular markers of hemangioma. Plast Reconstr Surg 106(3): 529-538. DOI: 10.1097/00006534-200009030-00001
- Delahunt B, Stehbens WE, Gilbert-Barness E, Shozawa T, Rüger BM (2000). Progeria kidney has abnormal mesangial collagen distribution. Pediatr Nephrol 15(3-4): 279-285. DOI: 10.1007/s004670000479
- Tan ST, Hasan Q, Velickovic M, Rüger BM, Davis RP, Davis PF (2000). A novel In Vitro human Model of Hemangioma. Modern Pathol 13(1):92-99. DOI: 10.1038/modpathol.3880014
- Greenhill NS, Rüger BM, Hasan Q, Davis PF (2000). The α1(VIII) and α2(VIII) Collagen Chains Form Two Distinct Homotrimeric Proteins in vivo. Matrix Biol 19(1): 19-28. DOI: 10.1016/s0945-053x(99)00053-0
- Kitching AR, Rüger BM, Davis PF (2000). Oxidant stress is increased within the glomerulus in experimental diabetic nephropathy. Nephrology 5(4): 263-270. https://doi.org/10.1046/j.1440-1797.2000.00011.x
- Rüger BM, Hasan Q, Erb KJ, Davis PF (1999). Progression of Renal Disease in Interleukin-4 Transgenic Mice: Involvement of Transforming Growth Factor- beta. Int J Exp Pathol 80(3): 113-123. DOI: 10.1046/j.1365-2613.1999.00105.x
- Erb KJ, Rüger B, von Breveren M, Ryffel B, Schimpl A, Rivett K (1997). Constitutive Expression of Interleukin (IL)-4 In Vivo Causes Autoimmune-type Disorders in Mice. J Exp Med 185(2): 329-339. DOI: 10.1084/jem.185.2.329
- Davis PF, He Y, Furneaux RH, Johnston PS, Rüger BM, Slim GC (1997). Inhibition of Angiogenesis by Oral Ingestion of Powdered Shark Cartilage in a Rat Model. Microvascular Res 54: 178-182. DOI: 10.1006/mvre.1997.2036
- Rüger B, Hasan Q, Greenhill NS, Davis PF, Dunbar PR, Neale TJ (1996). Mast Cells and Type VIII Collagen in Human Diabetic Nephropathy. Diabetologia 39: 1215-1222. DOI: 10.1007/BF02658509
- Neale TJ, Rüger B, Macaulay H, Dunbar PR, Hasan Q, Bourke A, Murray-McIntosh RP, Kitching RA (1995). Tumour necrosis factor-alpha is expressed by glomerular visceral epithelial cells in human membranous nephropathy. Am J Pathol 146: 1444-1454. PMID: 7778683
- Rüger BM, Sawada H, Dunbar PR, Hasan Q, Kittelberger R, Greenhill NS, Neale TJ (1994). Human mast cells produce type VIII collagen in vivo. Int J Exp Pathol 75: 397-404. PMID: 7734329
- Neale TJ, Ojha PP, Exner M, Poczewski H, Davis PF, Rüger B, Witztum J, Kerjaschki D (1994). Proteinuria in Passive Heymann Nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest 94: 1577-1584. DOI: 10.1172/JCI117499
Professional Experience, Appointments, Research
- October 1999–present:
- Department of Blood Group Serology and Transfusion Medicine, Integrative Cell Biology Research Laboratory, Medical University Vienna
- Research focus: Establishment of reparative 3D niche models
- April 1992–April 1999:
- Department of Medicine, University of Otago, Wellington, New Zealand, Immunopathology Research Laboratory
- Renal research, mast cell-, extracellular matrix- and angiogenesis research
- July 1984–March 1992:
- Institute of Clinical Pathology, University of Vienna, Haematopathology Laboratory
- Diagnostic immunohistochemistry of haematological disorders
- April 1984–June 1984:
- Ludwig Boltzmann Institute of Experimental Anaesthesia and Intensive Care, University of Vienna
- Endorphin research
- Jan 1982–March 1984
- 2nd Medical University Clinic, University of Vienna
- Prostaglandin research project
Education
- 2014–2021
- PhD programme Vascular Biology
- Department of Blood Group Serology and Transfusion Medicine, Medical University of Vienna, Austria
- Thesis: Neovascularization in the context of inflammation: Three-dimensional in vitro culture models to study postnatal vascular morphogenesis; Supervisor: Ao.Prof. Dr. Johannes M. Breuss
- 1996–1998
- Master of Medical Laboratory Science (MMLSc),
- University of Otago, Wellington, New Zealand
- Thesis: The roles of interleukin-4 and transforming growth factor-beta in the development of progressive renal disease
- Supervisors: Dr. Paul F. Davis, Prof. Dr. Linda Holloway
- 1979–1981
- School of Biomedical Laboratory Science, Vienna, Austria
International Collaborations
- Jan/Feb 1997
- Co-operative project under the NZ/FRG Scientific and Technological Cooperation (STC) Agreement Programme,
- Institute of Biochemistry, Cologne (Prof. Dr. Mats Paulsson)
- Preparation of beta-2 laminin fusion proteins for the production of chain-specific antibodies
Awards
- Best Poster Award, Bristol Meyer Squibb, March 1994:
- Australian and New Zealand Society of Nephrology (ANZSN) Scientific Meeting, Adelaide
- Rüger BM, Dunbar PR, Hasan Q, Greenhill NS, Sawada H, Neale TJ: Mast Cells and Type VIII Collagen in Human Diabetic Nephropathy
- Young Investigator Award, September 1996:
- International Society of Nephrology (ISN) Forefronts in Nephrology: Cytokines and Adhesion Molecules in Tissue Injury and Repair, Snowbird, Utah
- Rüger BM, Hasan Q, Davis PF, Pidgeon GB, Greenhill NS, Erb KJ: Interleukin-4 transgenic (IL-4 tg) mice: a new model of progressive glomerulosclerosis
- Best Abstract Award, September 2008:
- Deutsche Gesellschaft für Transfusionsmedizin und Immunhämatologie (DGTI), Düsseldorf, Germany:
- Beate M. Rüger, David Hollemann, Genya Yanagida, Michael B. Fischer, Markus Dettke (2008). Vascular morphogenesis by adult bone-marrow (BM) progenitor cells in three dimensional (3D) fibrin matrices
- 3D cell-culture
- Flow cytometry
- Immunocytochemistry
- Confocal laser scanning microscopy
- ELISA, Luminex, Protein Array
- Western Blotting
- RT-PCR
3D-Zellkulturen simulieren im Vergleich zu herkömmlichen 2D-Zellkulturen die in vivo Situation viel genauer, da die drei-dimensionale Anordnung Einfluss hat auf Zellfunktionen, Zell-Zell-Interaktionen und – Kontakte sowie Wechselwirkungen mit der extrazellulären Matrix. Der Fokus in unserem Labor ist die Erforschung zirkulierender Vorläuferzellen, die bei der Blutgefäßneubildung im Rahmen der Regeneration nach Gewebeschädigung maßgeblich beteiligt sind. Mit der Etablierung eines 3D Nischenmodells konnten wir zeigen, dass ein komplexes synergistisches Netzwerk aus zellulären, extrazellulären und parakrinen Interaktionen zwischen mesenchymalen Stromalzellen (MSC), hematopoetischen Vorläuferzellen und Immunzellen aus dem Blut zur de-novo-Bildung von Gefäßstrukturen durch Selbstorganisation führen kann.
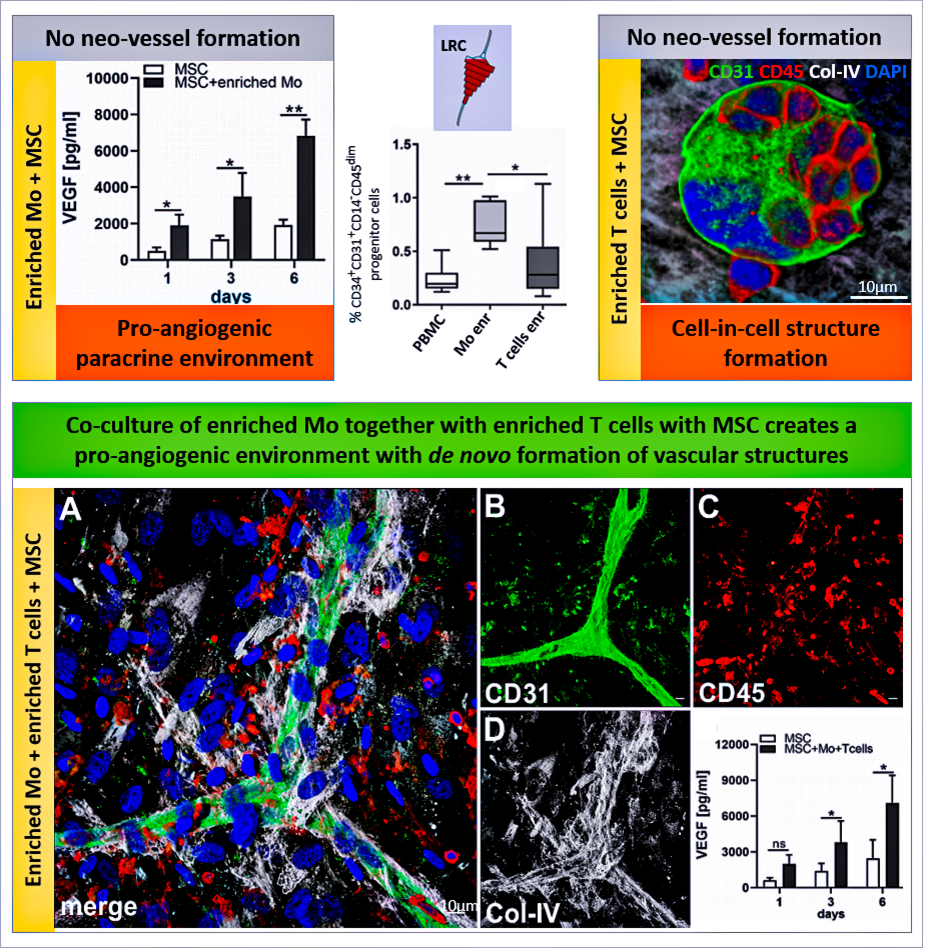
Leukozytenreduktionskammern (LRC), die während der Thrombozytenapherese erzeugt werden, eignen sich hervorragend zur Kultur von mononukleären Zellen (MNC) aus dem Blut und enthalten eine erhebliche Anzahl von hematopoietischen Vorläuferzellen. Die ko-Kultur von MSC und angereicherten Monozyten (Mo) zusammen mit angereicherten T-Zellen (und den darin enthaltenen hematopoetischen Vorläuferzellen) führt zu erhöhter VEGF-Sekretion und Bildung komplexer Gefäßstrukturen in 3D-Fibringelen. Veröffentlicht unter »DOI: 10.3389/fbioe.2020.602210.
Rüger BM, Buchacher T, Dauber E-M, Pasztorek M, Uhrin P, Fischer MB, Breuss JM, and Leitner GC (2020). »De novo Vessel Formation Through Cross-Talk of Blood-Derived Cells and Mesenchymal Stromal Cells in the Absence of Pre-existing Vascular Structures. Frontiers in Bioengineering and Biotechnology 8. DOI: 10.3389/fbioe.2020.602210